
|
|
|
60. ELECTROCHEMICAL MACHINING (ECM)
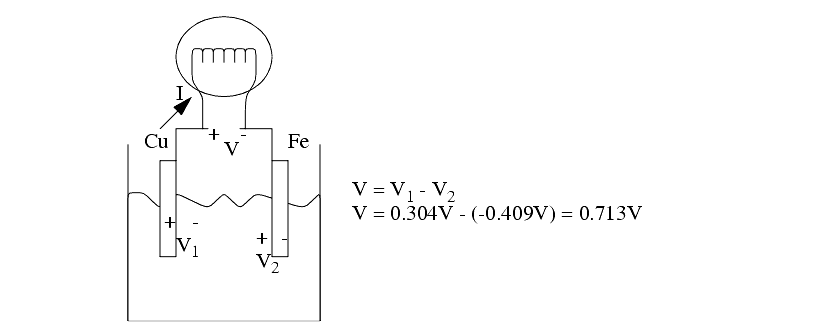
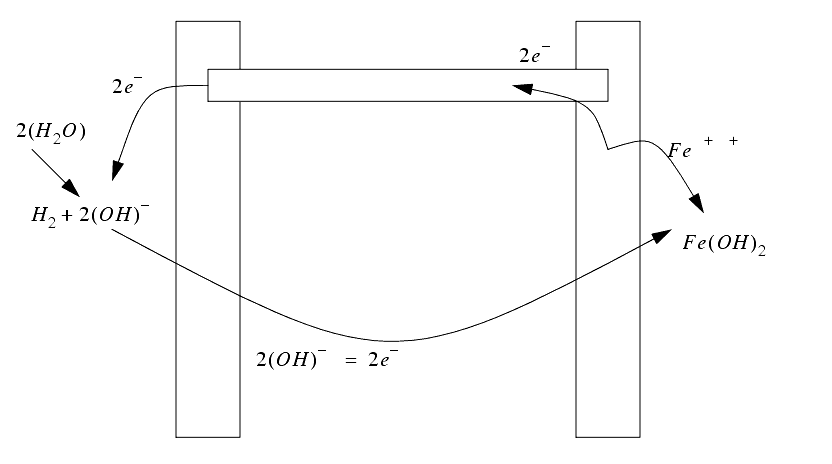
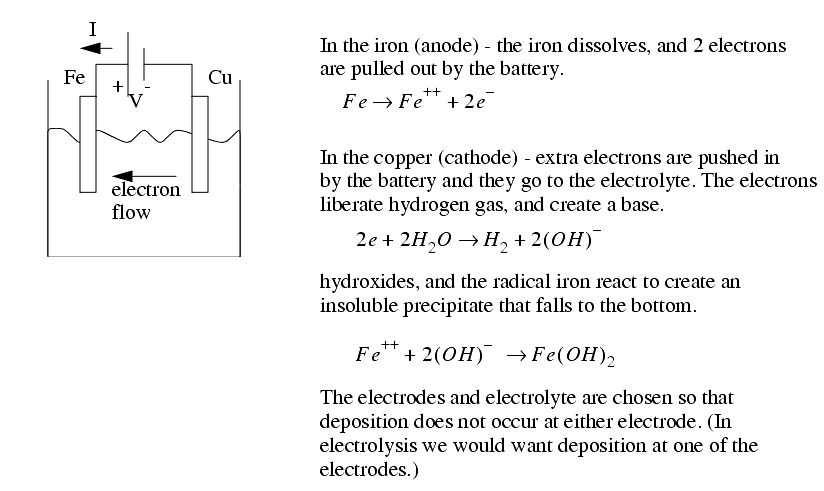
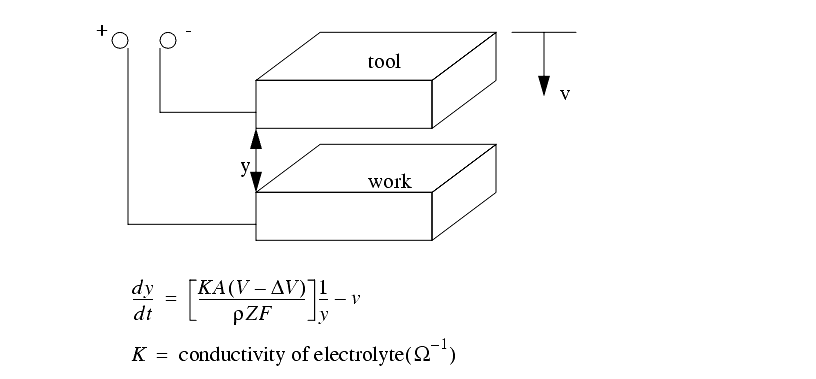
������������The physics - an electrode and workpiece (conductor) are placed in an electrolyte, and a potential voltage is applied. On the anode (+ve) side the metal molecules ionize (lose electrons) break free of the workpiece, and travel through the electrolyte to the electrode (a cathode; has a -ve charge; a surplus of electrons).
NOTE: in EDM an arc was used to heat metal, here the metal dissolves chemically.
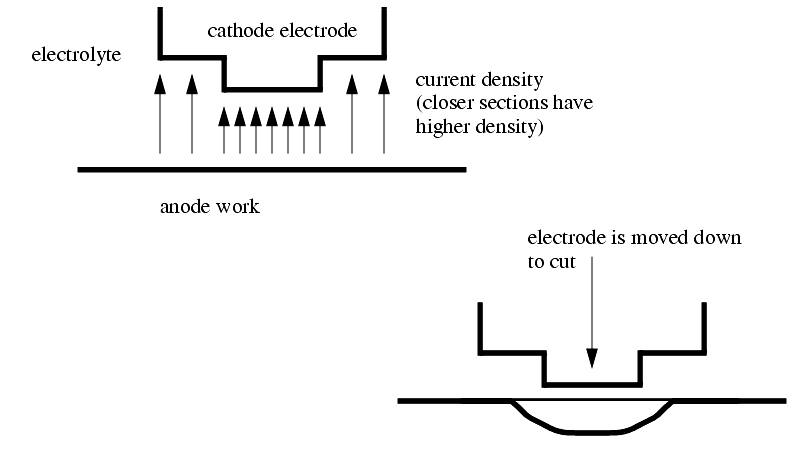
Variation in the current density will result in work taking the electrodes shape.
The electrode is fed with a constant velocity, and the electrolyte is fed through the tool. The tool is designed to eliminate deposition of the ionized metal on the electrode.
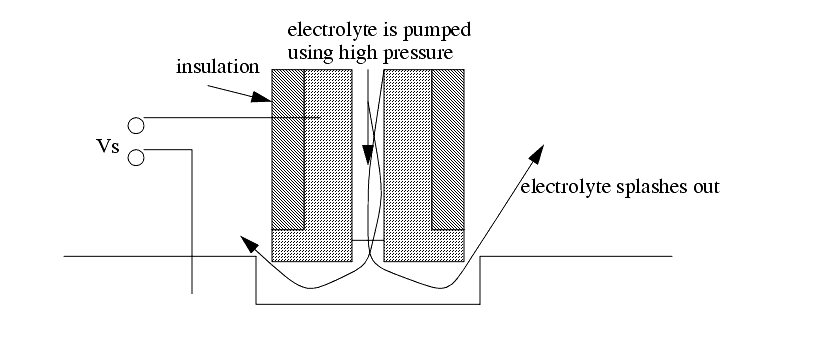
Supply V = 8 to 20V, I = >1000A.
Electrode gap is typically 0.1 to 0.2 mm.
mrr is about 1600mm3/min. per 1000A, OR 3KWhr for 16000 mm3 (not very efficient, 30 times more than standard machining techniques).
mrr is independent of material hardness.
Good for low machinability, or complicated shapes.
Forces are large with this method because of fluid pumping forces.

The basic principle is shown below
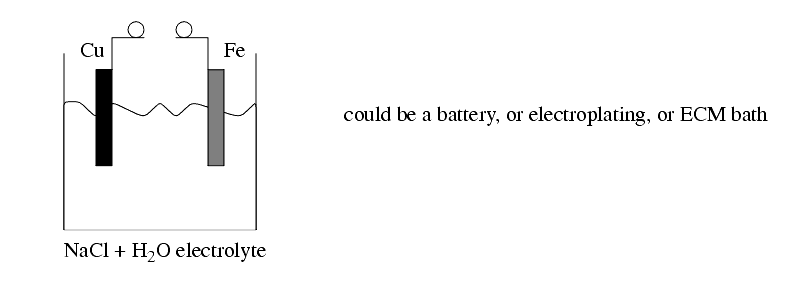
The chemical reaction between an electrode and the electrolyte leads to electrons being added, or removed from the electrode metal. This addition/subtraction leads to a voltage potential.
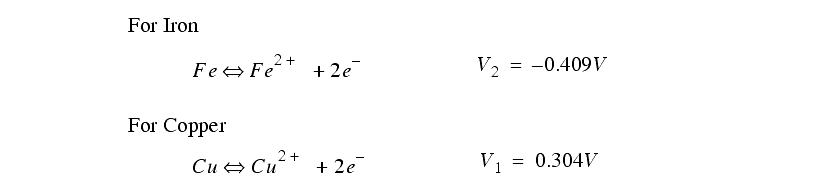
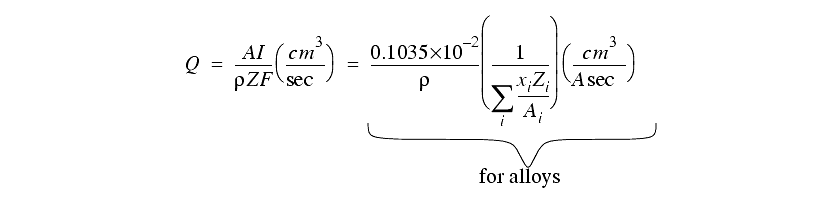
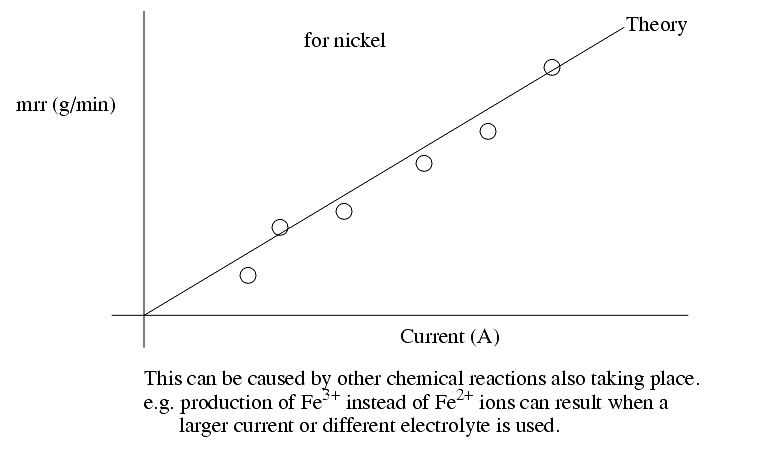
Actual rates may vary from theory as other factors come into effect.
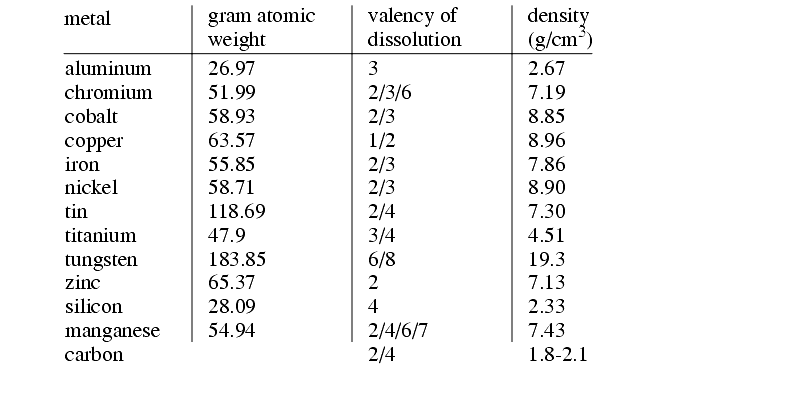
The table below shows various materials and relevant properties,
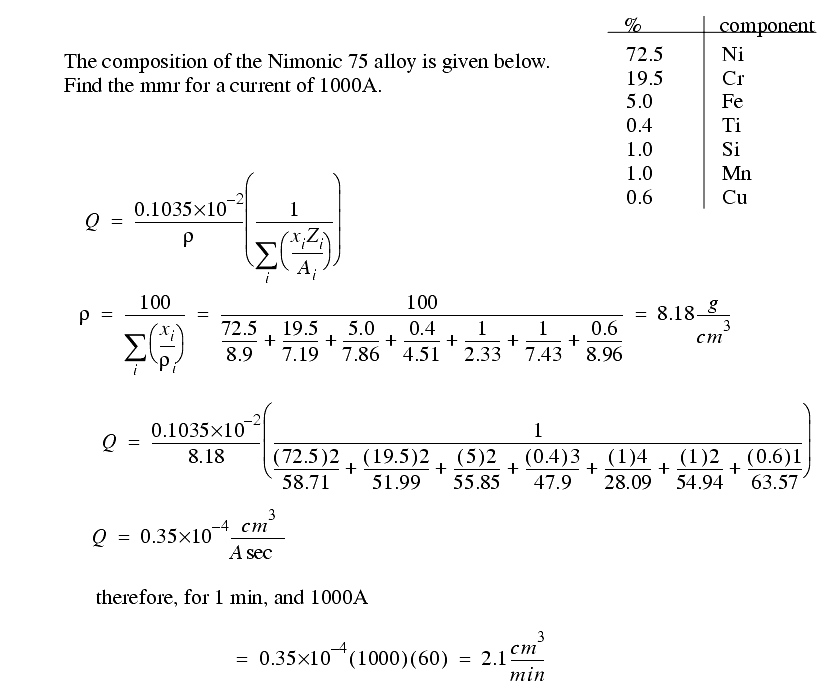
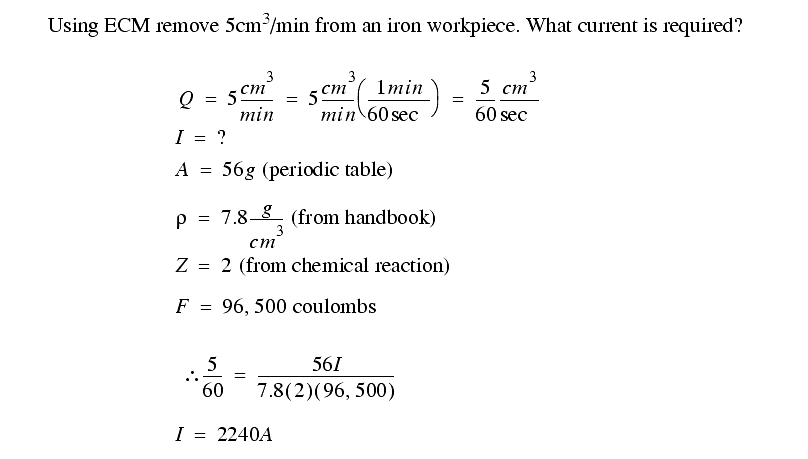
While the current required is related to the metal removed, the voltage required depends upon,
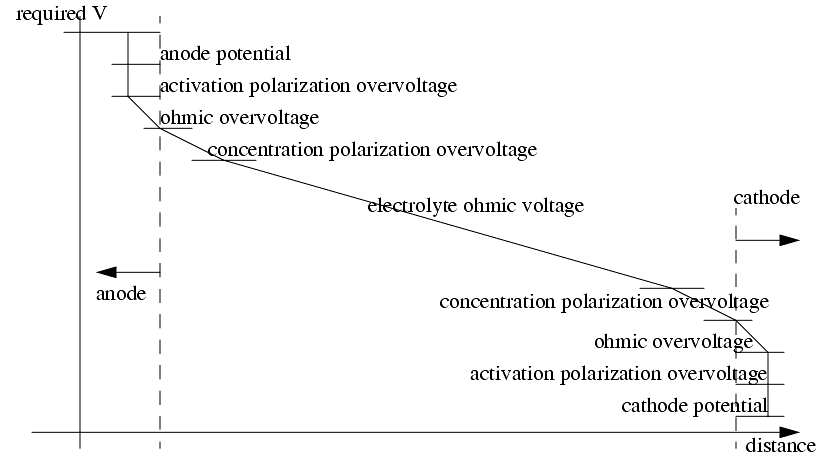
- the current flow in and about the electrodes will disturb the normal distribution of voltage. Extra potential is required to overcome the effects.
- Ion collect near electrodes and impede ion transfer from the electrode to the electrolyte, also adding a potential.
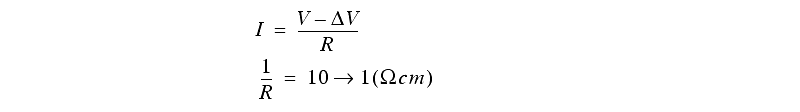
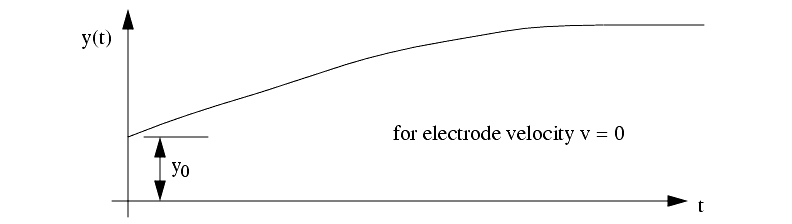
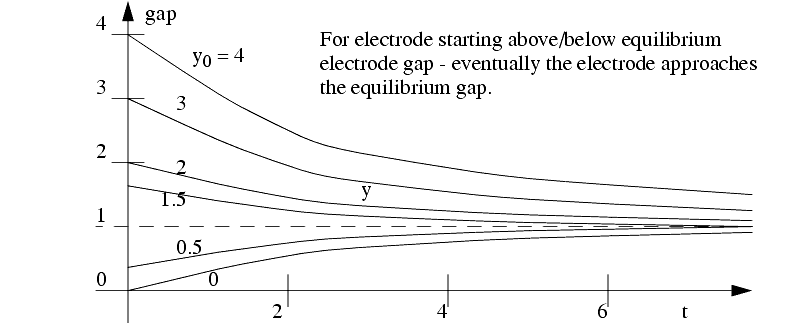
The feed of the electrodes has the following effects
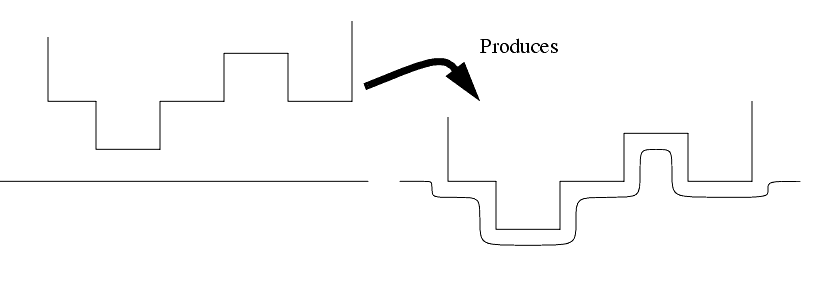
The ECM process will erode material in a radial direction, so care must be made in tooling design.
As current flows through the electrolyte, it is heated, and conductivity decreases.
Surface finish is affected by,

Summary of ECM characteristics,
- shape application - blind complex cavities, curved surfaces, through cutting, large through cavities.
- limitations - high specific energy consumption (about 150 times that required for conventional processes), not applicable with electrically non-conducting materials and jobs with very small dimensions, expensive machines.
This technique has been combined with a metal grinding wheel in a process called Electrolytic drilling. The wheel does not touch the work, and gives a surface finish from 8 to 20 min.
Search for More: |

Custom Search
|

|