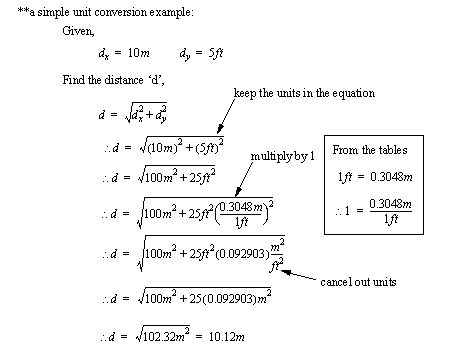
2.4.3 A Table
Distance
1 ft. (feet) = 12 in. (inches) = 0.3048 m (meter)
1 mile = 1760 yards = 5280 ft = 1.609km
1 in.(inch) = 2.540 cm
1 yd (yard) = 3 ft.
1 nautical mile = 6080 ft. = 1852 m = 1.150782 mi
1 micron = 10-6 m
1 angstrom = 10-10 m
1 mil = 10-3 in
1 acre = 43,560 ft. = 0.4047 hectares
1 furlong = 660 ft
1 lightyear = 9.460528e15 m
1 parsec = 3.085678e16 m
Area
1 acre = 43,559.66 ft2
1 Hectare (ha) = 10,000 m2
1 Hectare (ha) = 10,000 m2
1 Hectare (ha) = 10,000 m2
1 Hectare (ha) = 10,000 m2
Velocity
1 mph = 0.8689762 knot
Angle
1 rev = 2PI radians = 360 degrees = 400 gradians
1 degree = 60 minutes
1 minute = 60 seconds
Volume
1 US gallon = 231 in3
1 CC = 1 cm3
1 IMP gallon = 277.274 in3
1 barrel = 31 IMP gal. = 31.5 US gal.
1 US gal. = 3.785 l = 4 quarts = 8 pints = 16 cups
1 liter (l) = 0.001 m3 = 2.1 pints (pt) = 1.06 quarts (qt) = 0.26 gallons (gal)
1 qt (quart) = 0.9464 l
1 cup (c) = 0.2365882 l = 8 USoz
1 US oz = 8 US drams = 456.0129 drops = 480 US minim = 1.0408 IMP oz
= 2 tablespoons = 6 teaspoons
1 IMP gal. = 1.201 U.S. gal.
1 US pint = 16 US oz
1 IMP pint = 20 IMP oz
1tablespoon = 0.5 oz.
1 bushel = 32 quarts
1 peck = 8 quarts
Force/Mass
1 N (newton) = 1 kg•m/s2 = 100,000 dyne
1 dyne = 2.248*10-6 lb. (pound)
1 kg = 9.81 N (on earth surface) = 2.2046 lb
1lbf = 16 oz. (ounce) = 4.448N
1 oz. = 28.35 g (gram) = 0.2780N
1 lb = 0.03108 slug
1 kip = 1000 lb.
1 slug = 14.59 kg
1 imperial ton = 2000 lb = 907.2 kg
1 metric tonne = 1000 kg
1 troy oz = 480 grain (gr)
1 g = 5 carat
1 pennyweight = 24 grain
1 stone = 14 lb
1 long ton = 2240 lb
1 short ton = 2000 lb
Pressure
1 Pascal (Pa) = 1 N/m2 = 6.895 kPa
1 atm (metric atmos.) =760 mmHg at 0°C=14.223 lb/in2=1.0132*105 N/m2
1 psi = 2.0355 in. Hg at 32F = 2.0416 in. Hg at 62F
1 microbar = 0.1 N/m2
Scale/Magnitude
atto (a) = 10-18
femto (f) = 10-15
pico (p) = 10-12
nano (n) = 10-9
micro (μ) = 10-6
milli (m) = 10-3
centi (c) 10-2
deci (d) = 10-1
deka (da) = 10
hecto (h) = 102
kilo (K) = 103
mega (M) = 106
giga (G) = 109
tera (T) = 1012
peta (P) = 1015
exa (E) = 1018
Power
1 h.p. (horsepower) = 745.7 W (watts) = 2.545 BTU/hr. = 550 ft.lb./sec.
1 ft•lb/s = 1.356 W
1 J (joule) = 1 N•m = 107 ergs = 0.2389 cal.
1 W = 1 J/s
1 ev = 1.60219*10-19 J
1 erg = 10-7 J
Temperature
°F = [(°C*9)/5]+32, °C = Celsius (Centigrade), F = Fahrenheit
K = Kelvin
Rankine (R) = F - 459.666
0.252 calories = 1 BTU (British Thermal Unit)
-273.2 °C = -459.7 °F = 0 K = 0 R = absolute zero
0 °C = 32 °F = 273.3 K = 491.7 R = Water Freezes
100°C = 212°F = 373.3 K = 671.7 R = Water Boils (1 atm. pressure)
1 therm = 100,000 BTU
Mathematical
π radians = 3.1416 radians = 180 degrees = 0.5 cycles
1 Hz = 1 cycle/sec.
1 rpm (revolutions per minute) = 60 RPS (Revolutions per second) = 60Hz
1 fps (foot per second) = 1 ft/sec
1 mph (miles per hour) = 1 mi./hr.
1 cfm (cubic foot per minute) = 1 ft3/min.
e = 2.718
Time
1 Hz (hertz) = 1 s-1
1 year = 365 days = 52 weeks = 12 months
1 leap year = 366 days
1 day = 24 hours
1 fortnight = 14 days
1 hour = 60 min.
1 min = 60 seconds
1 millenium = 1000 years
1 century = 100 years
1 decade = 10 years
Physical Constants
R = 1.987 cal/mole K = ideal gas law constant
K = Boltzmann’s constant = 1.3x10-16 erg/K = 1.3x10-23 J/K
h = Planck’s constant = 6.62x10-27 erg-sec = 6.62x10-34 J.sec
Avagadro’s number = 6.02x1023 atoms/atomic weight
density of water = 1 g/cm3
electron charge = 1.60x10-19 coul.
electron rest mass = 9.11*10**-31 Kg
proton rest mass = 1.67*10**-27 Kg
speed of light (c) = 3.00x1010 cm/sec
speed of sound in dry air 25 C = 331 m/s
gravitational constant = 6.67*10**-11 Nm**2/Kg**2
permittivity of free space = 8.85*10**-12 farad/m
permeability of free space = 1.26*10**-6 henry/m
mean radius of earth = 6370 Km
mass of earth = 5.98*10**24 Kg
Electromagnetic
magnetic flux = weber (We) = 10**8 maxwell
inductance = henry
magnetic flux density = tesla (T) = 10**4 gauss
magnetic intensity = ampere/m = 0.004*PI oersted
electric flux density = coulomb/m**2
capacitance = farad
permeability = henry/m
electric field strength = V/m
luminous flux = lumen
luminance = candela/m**2
1 flame = 4 foot candles = 43.05564 lux = 43.05564 meter-candles
illumination = lux
resistance = ohm